Pharmaceutical Company Explores Market Opportunities in Breast Cancer
Posted by | Fuld & Company , Fuld & Company
 Background & Challenge
Background & Challenge
A pharmaceutical client in the oncology market was in need of effectively tracking the developmental programs of the metastatic breast cancer market.
Approach
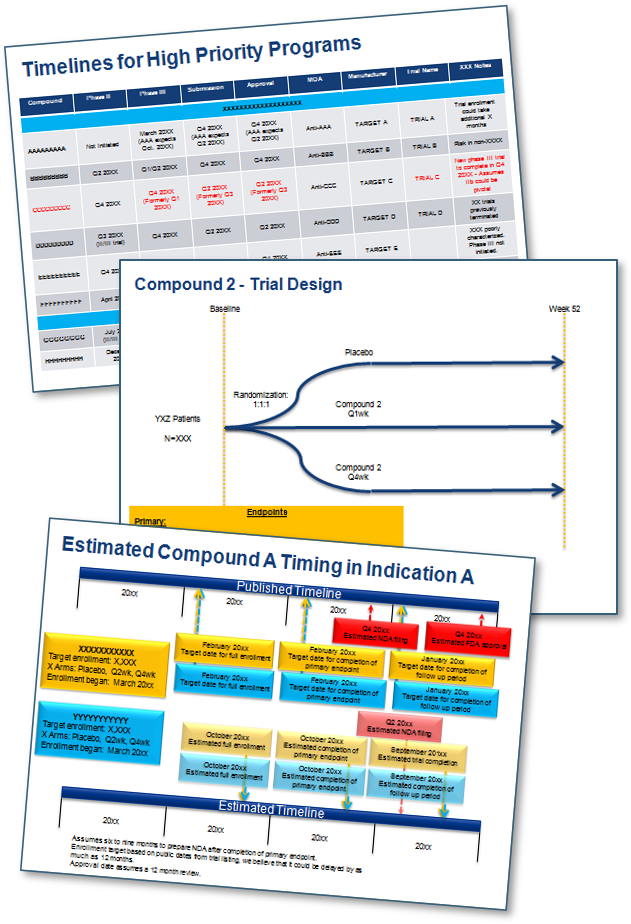
- Created a clinical development pipeline of all early to late-stage therapies, providing insights into the clinical trial design, target indication, and primary and secondary endpoints.
- Prioritized key competitors in the market, and created data readouts, filing, and market launch timelines based on primary and secondary research.
- For selected on-market therapy, determined commercialization activity, and strategy.
- Attended ASCO and SABC on behalf of the client.
- Updated the client by e-mail of any new key findings and market updates.
- Provided the client with quarterly detailed findings using graphics and charts in easy to understand formats.
Result & Benefits
Client received regular and timely updates on developments and changes in its competitors’ programs using its corporate templates. Fuld identified competitor product delays allowing the client to significantly revise its corporate earnings forecast. Fuld detailed specific marketing strategies that allowed clients to counter strategize. Fuld also identified potential partnering and in-licensing opportunities.
[gravityform id=”48″ title=”true” description=”true”]
Tags: BioPharma, Competitive Intelligence, Diagnostics, Healthcare & Life Sciences, Mergers & Acquisition, Pharmaceuticals